Background:
Despite chimeric antigen receptor (CAR) T-cell therapy has achieved great advances in recent year, approximately 50% of relapsed/refractory B cell acute lymphoblastic leukemia (r/r B-ALL) patients treated with CAR-T experience relapse 6 months post CAR-T treatment. CD20 express on 30 to 50% of B-ALL, which makes CD20 Monoclonal Antibody as one of the potential therapy strategies to decrease the tumor burden and improve the efficacy of CAR-T therapy. Adding Rituximab to chemotherapy protocol had been demonstrated to improve the outcome for CD20-positive ALL. However, rare study explored the influence of Rituximab combined with CAR-T therapy.
Methods:
We retrospectively analyzed 20 r/r B-ALL patients who received CAR-T therapy, all of whom had failed multiple lines of therapy. Before CAR-T infusion, we administered Rituximab to 10 patient with high CD20 expression at a dose of 375mg/m2 for 1 day. Meanwhile, we selected 10 patients with the comparable features who underwent CAR-T treatment without Rituximab in the same period as the control group. In vitro, the surface molecule expression and killing of CAR-T post Rituximab-treated B-ALL cells co-incubated with CAR-T cells were detected by flow cytometry.
Results
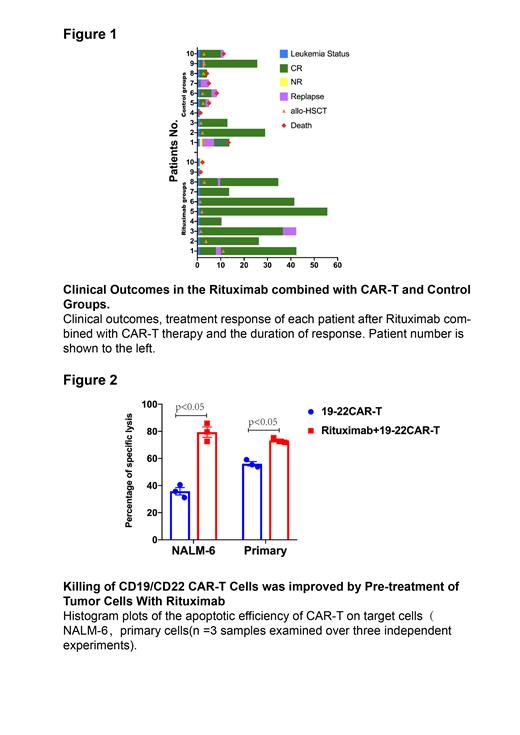
The day 28 after CAR-T cells infusion, 8(80%) and 6(60%) of the 10 patients achieved minimal residual disease negative complete remission (MRD-negative CR) in Rituximab group and Control group, respectively. The median follow-up of Rituximab and Control groups were 29.27 and 9.83 months. The 2-year overall survival (OS) and leukemia-free survival (LFS) rates both were longer in the Rituximab group (90% vs 26.7%, p=0.034; 41.7% vs 25%, P=0.14). Particularly, subgroup analysis showed that Rituximab could markedly improve the OS and LFS for relapsed patients (88.9% vs 25%, P=0.0203; 87.5% vs 15%, P=0.0112). In addition, CAR-T combined with Rituximab improved long-term outcome in patients who had failed multiple lines of therapy (>5) (2-year OS 100% vs 16.7%, P=0.0205; 1-year LFS 50% vs 0%, P=0.011). There was no significant difference between the two groups in hematological toxicities as well as in non-hematological toxicities. All adverse events were manageable(Figure1).
In vitro, we observed that Rituximab-treated tumor cells are more sensitive to CAR-T killing and a broad range of cytokines and chemokines were produced when Rituximab-treated Nalm-6 cells co-cultured with 19-22CAR-T cells, such as interferon-γ(IFN-γ), tumor necrosis factor-α (TNF-α) and interleukin-2 (IL-2)(IL-2:272.5 pg/ml vs 84.83 pg/ml, P=0.0243; IFN-γ: 6111 pg/ml vs 796 pg/ml, P=0.0451,TNF-α: 1374 pg/ml vs 629.4 pg/ml, P=0.048)(Figure 2). To investigate whether Rituximab has an effect on CAR-T persistence, we stimulated CAR-T cells repeatedly in vitro with Rituximab-treated Nalm-6 to evaluate the changes in CAR-T surface exhaustion molecules at different times. We found that the expression of exhaustion molecules (LAG-3, PD-1, TIM-3) on CAR-T cells were significantly lower in the Rituximab group than in the Control group (LAG-3: 57.23% vs 71.8%, P=0.0015; PD-1: 35.57% vs 69.03%, P<0.001; TIM-3:36.53% vs 68.9%, P<0.001).
Conclusion:
Rituximab combined with CAR-T therapy was effective for improving the long-term outcome of B-ALL patients who have failed multiple lines of therapy. In vitro, we observed that rituximab potentially improved CAR-T efficacy by sensitizing Leukemia Cells to CAR T-mediated cytotoxicity and reducing CAR-T exhaustion.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal